The 3C Trial Protocol
Below we give a summary of the trial protocol. If you wish, you may download the full version of the protocol as a pdf from the following link:
3C Trial Protocol Version 5 PDF
Campath, Calcineurin inhibitor reduction and Chronic allograft nephropathy
Protocol Summary
Background
The major challenge facing the kidney transplantation community at present is improving the long-term survival of grafts. Although there has been significant progress in reducing acute rejection in the last decade, there has been no concomitant increase in the long-term survival of kidney transplants.
Calcineurin inhibitors (CNIs, ciclosporin and tacrolimus) have improved acute rejection rates, but also contribute significantly to chronic allograft nephropathy (CAN, the commonest cause of late graft loss). Efforts have been made to reduce exposure to these agents, but these are often hampered by an increased incidence of acute and chronic rejection. Promising methods of reducing exposure to CNIs have been using ‘induction’ therapy at the time of transplantation (antibody-based treatments which dampen the immune response to the graft in the period immediately following the operation when the risk of rejection is highest) and using alternative maintenance immunosuppressants in the place of CNIs.
Campath-1H (alemtuzumab) is a humanized monoclonal antibody against CD52, which is expressed on many immune cells, and two doses around the time of surgery rapidly deplete the body of lymphocytes (the main immune cell responsible for rejection). This powerful ‘induction’ therapy allows a significant reduction in the amount of maintenance immunosuppression required, and therefore limits the exposure to CNIs. Sirolimus can be used for maintenance immunosuppression, but is not a CNI and is not nephrotoxic. Many trials have shown that switching patients from a CNI-based regimen to sirolimus has a beneficial effect on the function of the graft, which could well translate into a prolonged lifespan. Furthermore, both Campath-1H and sirolimus have potentially beneficial effects on the immune system, such that they may induce a state of near ‘tolerance’, in which the host immune system requires much less suppression to prevent it from rejecting the transplant.
The 3C study will investigate both of these approaches, and elucidate whether using Campath-1H induction with or without a switch to sirolimus-based immunosuppression improves the function, and long-term survival, of kidney transplants.
Design
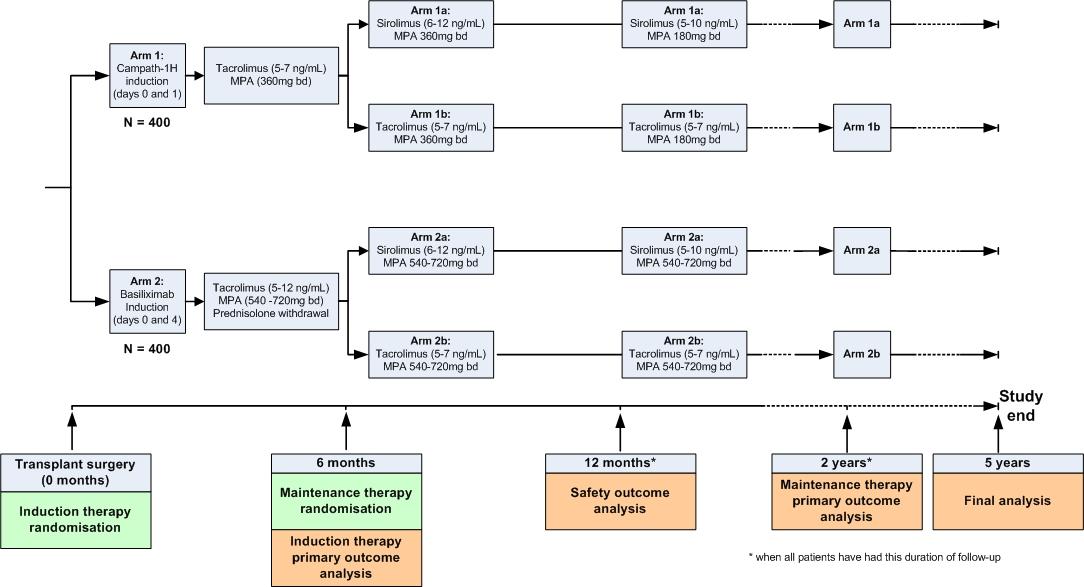
Aim and Assessments
The 3C study will include 800 patients aged over 18 years old who have been listed for kidney transplantation. The primary aims are to assess the effects of allocation to:
- Campath-1H based induction versus basiliximab based induction on biopsy-proven acute rejection at six months; and
- sirolimus-based maintenance therapy versus tacrolimus-based maintenance therapy on graft function at 2 years.
Secondary assessments will include the effect of study treatments on
- graft-related outcomes (graft survival, acute rejection);
- safety outcomes (infections, malignancy, patient survival);
- treatment-related side-effects (diabetes mellitus, bone marrow suppression).
At the time of surgery, patients will be randomly allocated (using a minimization algorithm) to receive either Campath-1H or basiliximab induction. They will be allocated to their long-term maintenance therapy (sirolimus- or tacrolimus-based) by a second randomisation at six months after surgery.
Summary of Practical Procedures
Potentially eligible:
- active on transplant waiting list, or live donor transplant planned
- aged over 18
- male or female
Identification and Invitation
- potentially eligible patients identified from local transplant waiting list
- agreement to screen such patients sought from consultants
- patient sent Patient Information Sheet
Randomisation visit (prior to surgery)
- Written informed consent sought from eligible and willing patients
- Basic medical history and details of transplant recorded
-
Randomisation
- induction therapy: CAMPATH- or basiliximab-based induction
- Inform patient’s nephrologist, transplant surgeon and GP of randomisation
Discharge visit, and followup visits at 1, 3, 6, and 12 months
- Further details of medical history and transplant
- Serious adverse events
- Current medication, clinical measurements and laboratory results
- at 6 month visit: randomisation between sirolimus- or tacrolimus-based maintenance therapy
Followup after one year, six monthly until 6 years after surgery
- Postal questionnaire for serious adverse events and medications
- Registry sources for laboratory results, survival and cancers.
Monitoring of safety and efficacy
- Central review of blood results and SAEs by Coordinating Centre
- Further details on relevant outcomes from hospital records sought by LCC clinic staff
- Relevant events confirmed and reviewed by central Outcomes Adjudication Panel
